Pfizer Biontech Unterschied
2 days agoThe Pfizer-BioNTech COVID-19 vaccine is now available for children 5 to 11 years old in Washington state. Bei dem Mittel von Biontech und Pfizer handelt es sich um einen sogenannten mRNA-Impfstoff.
Sahin 55 and Tureci 53 set up BioNTech in the central German city of Mainz in 2008.

Pfizer biontech unterschied. During the clinical development stage BioNTech and its partners will provide clinical supply of the vaccine from its GMP-certified mRNA manufacturing facilities in Europe. BioNTechPfizers COVID-19 vaccine is currently authorized for children between the ages of 5 and 11 years old and teens between the ages of 12 and 15 and it is fully approved for. BioNTech is the Marketing.
Excluding Comirnaty 1 Revenues Grew 7 Operationally to 111 Billion Third-Quarter 2021 Reported Diluted EPS 2 of. ˈ f aɪ z ər FY-zər is an American multinational pharmaceutical and biotechnology corporation headquartered on 42nd Street in Manhattan New York CityThe company was established in 1849 in New York by two German immigrants Charles Pfizer 1824-1906 and his cousin Charles F. The Ministry of Health MOH said in a media release on Wednesday 23 June that these vaccines have the same research name - BNT162b2 - as the Pfizer.
Die FDA untersuchte Daten von mehr als. Folgenden Unterschied haben die beiden Impfstoffe. Pfizer-BioNTech COVID-19 vaccine is authorized for use in children and adolescents aged 1215 years and is licensed by the Food and Drug Administration FDA for persons aged 16 1.
COMIRNATY is the first COVID-19 vaccine to be granted FDA approval Approval is based on a comprehensive submission package including six-month efficacy and safety data after second dose More than 12 billion Pfizer-BioNTech doses have been delivered to more than 120 countries or territories around the world since December 2020 Pfizer Inc. The Pfizer-BioNTech COVID-19 Vaccine which is based on BioNTechs proprietary mRNA technology was developed by both BioNTech and Pfizer. And BioNTech SE received authorization from the US.
Ad Visit the Comirnaty COVID-19 Vaccine mRNA Consumer Website. Moderna will 2021 etwa 600 Millionen Impfdosen produzieren die EU hat sich derzeit davon 160 Millionen gesichert. Von BioNTechPfizer sind für dieses Jahr 300 Millionen Dosen bestellt.
Governments registry of clinical trials on April 30 2020the same day as the first-in-human vaccine. The BioNTech-Pfizer first-in-human vaccine study appeared on the US. On Monday the companys partner US pharmaceutical giant Pfizer said their candidate vaccine was.
BioNTech SE is a German biotechnology company based in Mainz that develops and manufactures active immunotherapies for patient-specific approaches to the treatment of diseases. 2021 wurde der Pfizer-BioNTech-Impfstoff von der FDA für Menschen ab einem Alter von 16 Jahren vollständig zugelassen und erhielt den Namen Comirnaty. Pfizer vaccine has been authorized for children ages 5-11.
Ad Visit the Comirnaty COVID-19 Vaccine mRNA Consumer Website. Governments commitment for free access for COVID-19 vaccines Pfizer and BioNTech. Moderna schützt besser vor schwerer Erkrankung als Biontech.
BioNTech and Pfizer intend to initiate the first clinical trials as early as the end of April 2020 assuming regulatory clearance. What to Tell Your Healthcare Provider Before Your Vaccination. The Food and Drug Administration said that it studied the vaccine safety in approximately.
SINGAPORE Singapore will soon be receiving a new batch of COVID-19 vaccines called Comirnaty which is the same as the Pfizer-BioNtech vaccines used in the national vaccination programme. FDA for emergency use of the Pfizer-BioNTech COVID-19 Vaccine for children 5 through 11 years of age. What to Tell Your Healthcare Provider Before Your Vaccination.
Third-Quarter 2021 Revenues of 241 Billion Reflecting 130 Operational Growth. Bei Patienten mit Biontech-Impfung sank der Impfschutz von 91 Prozent in den ersten vier Monaten auf 77 Prozent. At this time however the King County sites listed on our Getting vaccinated page do not yet have appointments available for ages 5.
Die Impfstoffe enthalten unterschiedliche. A bandage is seen on a childs arm after she received a Pfizer-BioNTech Covid-19 vaccine on November 3 2021 in Shoreline Wash. The state Department of Health DOH expanded COVID-19 vaccine eligibility.
Approximately 96 COVID-19 vaccines are at various stages of clinical development1 At present we have the interim results of four studies published in scientific journals on the PfizerBioNTech. Es gibt nur wenige Unterschiede zwischen den beiden Impfstoffen von PfizerBioNTech und Moderna. 2 days agoThe Pfizer-BioNTech shots have shown 907 efficacy in preventing COVID-19 in children aged 5-11.
Government placed an initial order of 100 million doses for 195 billion and can acquire up to 500 million additional doses Americans to receive the vaccine for free consistent with US. Bislang wurde noch kein Impfstoff dieser Art für den Menschen zugelassen.

Sars Cov 2 Schutzwirkung Von Bnt162b2 Lasst Etwas Schneller Nach Als

Corona Impfung Deutschland Uberblick Und Vergleich Zum Impfen Welche Impfstoffe Gibt Es Wirkung Gegen Mutationen Und Neue Vakzine Sudwest Presse Online

Unterschiede Und Gemeinsames Corona Impfstoffe Von Biontech Und Moderna Im Vergleich Welcher Wirkt Besser Shz De
Biontech Pfizer Versus Moderna Die Zugelassenen Mrna Impfstoffe Im Vergleich

Comirnaty Und Co Das Sind Die Namen Der Corona Impfstoffe Pandemie Die Rheinpfalz

Unterschiede Biontech Moderna Astrazeneca Johnson Johnson

Live Blog Moderna Impfstoff In Tubingen Angekommen Unterschiede Zu Biontech
Corona Impfstoffe Im Vergleich So Unterscheiden Sie Sich Ndr De Ratgeber Gesundheit

Biontech Moderna Astra Zeneca Corona Impfstoffe Im Vergleich

Wirkung Verfugbarkeit Preise Was Uber Die Impfstoffe Gegen Das Coronavirus Bekannt Ist Wissen Tagesspiegel
Biontech Moderna Astrazeneca Corona Impfstoffe Im Vergleich

Corona Impfung Was Unterscheidet Die Impfstoffe Von Moderna Und Biontech Pfizer Wissen

Die Corona Impfstoffe Im Vergleich

Moderna Schutzt Vielleicht Besser Gegen Delta Infektion Br24

Coronavirus Vakzine Die Vier Zugelassenen Impfstoffe Im Vergleich Forschung Lehre
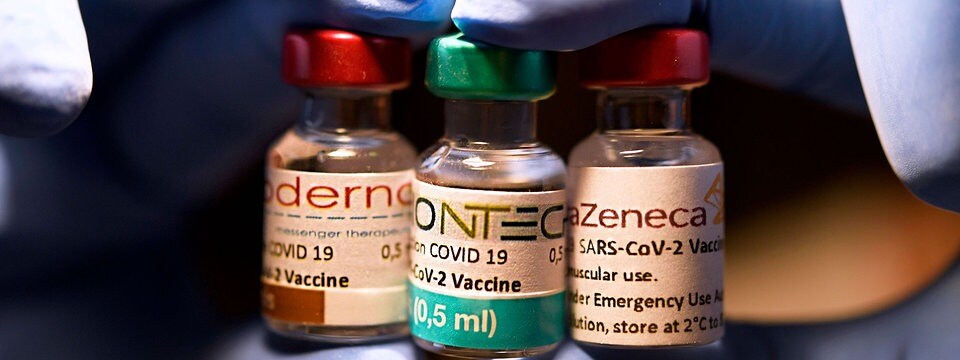
So Unterscheiden Sich Die Corona Impfstoffe Von Biontech Pfizer Und Moderna Mdr De

Corona Impfung Deutschland Impfstoffe Gegen Coronavirus Im Vergleich Biontech Moderna Astrazeneca Und Johnson Sudwest Presse Online